Temperature Measurement sneaks into your life like an uninvited guest and suddenly runs the whole house. It tells you what to wear, how long your coffee stays drinkable, and whether your phone decides to overheat and panic. One minute you’re shivering under a blanket, the next you’re arguing with the thermostat like it has feelings. Yet despite how often we complain about it, temperature rarely gets the respect it deserves. It isn’t just about hot days or cold nights. It’s science, motion, and measurement quietly working behind the scenes. Once you understand temperature properly, everyday numbers start making sense instead of just causing arguments.
Universe Converter
Universe Converter
Length, weight, time, speed, data & more — all in one orbit.
What Is Temperature?
At its core, temperature measures how fast particles move inside a substance. When particles move faster, temperature rises. When motion slows, temperature drops. This movement applies to solids, liquids, and gases alike. Even in a solid object that looks still, atoms vibrate constantly. Temperature reflects the average intensity of that vibration.
From a scientific standpoint, temperature represents the average molecular motion or molecular kinetic energy of particles. It does not measure how much energy an object contains overall. That distinction matters more than most people realize.
A small spark can reach an extremely high temperature, yet it carries very little energy. A bathtub of warm water has a lower temperature, but it contains far more energy. Temperature describes motion, not quantity.
Scientists also classify temperature as an intensive property. That means it does not depend on how much material is present. A spoonful of boiling water and a full pot of boiling water share the same temperature even though their total heat content differs greatly.
Volume Measurements A Complete Practical Guide
Temperature vs Heat A Critical Difference
Confusion between heat and temperature causes mistakes in learning, cooking, and even industrial work. Temperature tells how hot or cold something is. Heat describes energy transfer caused by temperature differences.
When you touch a hot surface, heat flows from the surface into your skin because of the temperature difference. The temperature indicates direction. Heat explains the movement of energy. Mixing these ideas leads to incorrect conclusions, especially in physics and chemistry.
Understanding the difference between temperature vs heat also explains why large objects at moderate temperature can feel more dangerous than small objects at extreme temperature. Energy transfer depends on mass and material, not temperature alone.
How Temperature Is Measured
Temperature measurement works by observing physical properties that change predictably with thermal motion. Instruments translate those changes into readable values. Over time, science refined these methods to improve accuracy and reliability.
Traditional thermometers rely on expansion. Mercury or alcohol expands as temperature rises and contracts as it falls. The visible movement inside a glass tube represents temperature change. Modern digital thermometers measure electrical resistance, which shifts with temperature in a predictable way.
Infrared temperature measurement detects thermal radiation emitted by objects. This allows measurement without physical contact. It proves essential in medicine, industrial safety, and environments where direct contact is impossible.
In industrial and scientific settings, thermocouples dominate. They generate a voltage when two different metals experience a temperature difference. This method remains reliable across extreme temperature environments.
Measurement accuracy matters. A difference of a few degrees can spoil food, damage electronics, weaken metal structures, or mask medical conditions. That is why calibration of thermometers plays a critical role in laboratories and industry.
Time calculator A Practical Guide to Measurement
Why Temperature Scales Exist
All temperature scales measure the same physical reality, but they use different reference points. Each scale answers the same question in a way that suits its audience. Some scales focus on everyday convenience. Others prioritize scientific accuracy.
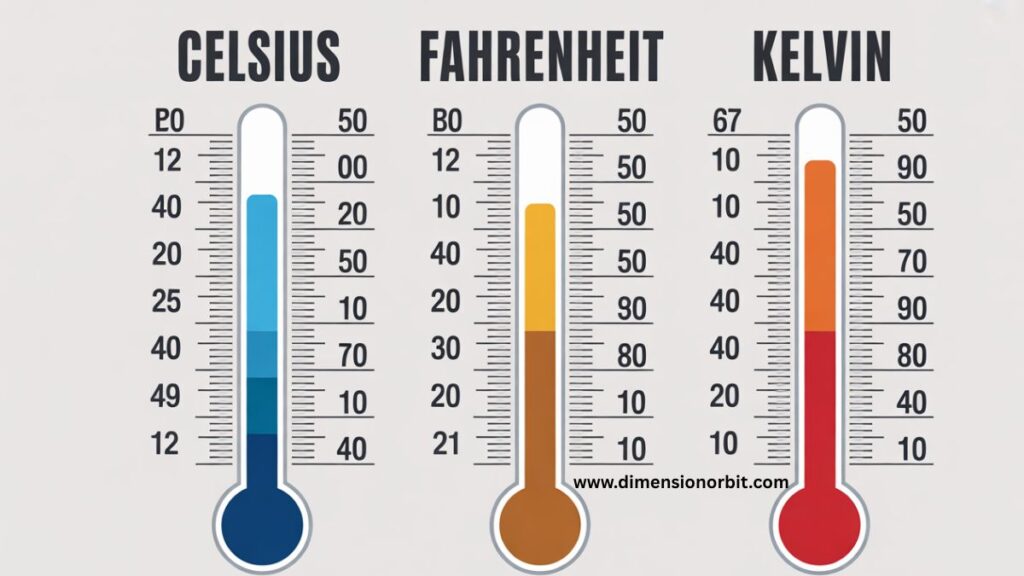
The three dominant types of temperature scales are Celsius, Fahrenheit, and Kelvin. Their differences lie in zero points, increment size, and purpose.
Celsius Temperature Scale
The Celsius temperature scale dominates daily life across most of the world. It anchors temperature to water behavior, which makes it intuitive and practical.
Zero degrees Celsius marks the freezing point of water. One hundred degrees Celsius marks the boiling point of water at standard atmospheric pressure. These reference points align naturally with daily experience, weather patterns, and cooking practices.
Celsius fits neatly into the metric system, which explains its widespread adoption in education, medicine, environmental science, and weather reporting. When people talk about a hot day, a cold winter, or a mild fever, Celsius communicates clearly.
Despite its practicality, Celsius does have limits. It does not represent absolute molecular stillness. Zero Celsius does not mean zero energy or zero motion. For advanced physics and chemistry, this limitation matters.
Live Speed measuring & calculation
Fahrenheit Temperature Scale
The Fahrenheit temperature scale often feels awkward to outsiders, yet it excels at describing human comfort. Its smaller degree increments provide finer resolution for everyday temperature changes.
In Fahrenheit, water freezes at thirty two degrees and boils at two hundred twelve degrees. These numbers appear arbitrary, but the scale spreads common weather temperatures across a wider numeric range. This makes small temperature changes feel more noticeable in daily life.
That precision explains why Fahrenheit remains common in weather forecasts and household thermostats in the United States. A difference of five degrees Fahrenheit often feels meaningful to human skin, especially within the typical comfort range.
While Fahrenheit lacks scientific elegance, dismissing it entirely ignores its practical strength in comfort-focused contexts.
Kelvin Temperature Scale
The Kelvin temperature scale forms the backbone of modern science. Unlike Celsius and Fahrenheit, Kelvin starts at absolute zero temperature, the point where molecular motion reaches its minimum possible value.
Absolute zero equals zero Kelvin. At this point, particles stop moving as much as the laws of physics allow. Kelvin does not use a degree symbol because it measures absolute thermal state, not relative increments based on human experience.
Kelvin matches the Celsius scale in increment size, which simplifies conversions. Adding 273.15 to a Celsius value converts it directly into Kelvin.
Scientists use Kelvin because scientific equations require an absolute temperature reference. Using Celsius or Fahrenheit in thermodynamic calculations produces incorrect results. Kelvin prevents that problem and ensures consistency across physics, chemistry, and engineering.
Comparing Celsius, Fahrenheit, and Kelvin
Each temperature scale exists for a reason. Celsius supports everyday communication and education. Fahrenheit refines human comfort perception. Kelvin anchors scientific truth.
No scale replaces the others completely. Choosing the right scale depends on context. Weather reports, medical readings, industrial processes, and scientific research all demand different approaches to temperature measurement.
Temperature Conversion Without Confusion
Temperature conversion often trips people up because scales do not align perfectly. Celsius and Fahrenheit use different zero points and increment sizes. Kelvin introduces absolute reference.
Understanding the logic behind conversion matters more than memorizing formulas. Celsius and Kelvin differ only by an offset. Fahrenheit involves both scaling and shifting. Many conversion errors happen when people forget that temperature scales are not linear copies of one another.
Clear understanding prevents mistakes in laboratories, classrooms, and real-world applications.
Real-World Applications of Temperature Measurement
Temperature measurement touches nearly every field. In weather forecasting, consistent temperature data allows scientists to track climate patterns and extreme events. Medicine, body temperature measurement helps diagnose infections and metabolic disorders.
In food safety, temperature controls bacterial growth. Cooking and refrigeration depend on precise thermal limits. In engineering, materials expand, contract, harden, or fail depending on temperature conditions. Industrial temperature control protects both products and people.
Extreme environments push temperature measurement to its limits. Space exploration, deep-sea research, and cryogenic technology rely on accurate readings far beyond human experience.
Why Different Fields Use Different Scales
Scientific disciplines require absolute accuracy, which explains their reliance on Kelvin. Daily life values intuition and convenience, which favors Celsius or Fahrenheit depending on cultural context. History also plays a role. Measurement systems persist long after their original rationale fades.
Global temperature standards exist, yet complete standardization remains unlikely. Human habits change slowly, especially when comfort and familiarity feel at stake.
Common Misunderstandings About Temperature
Negative temperatures do not mean the absence of energy. Particles still move. Kelvin cannot go below zero because absolute zero represents the lowest possible thermal state. Fahrenheit is not outdated; it simply serves a different purpose. Celsius does not replace Kelvin in scientific work.
Clearing up these misunderstandings improves both scientific literacy and everyday decision-making.
Choosing the Right Temperature Scale
Celsius works best for daily life, education, and health contexts. Fahrenheit communicates comfort levels effectively in weather discussions. Kelvin remains essential for science, engineering, and research.
Using the correct scale avoids errors, miscommunication, and flawed calculations.
Digital tool
Digital tools like Feet and Inches Calculator can convert inches to centimeters or feet instantly. Many smartphone apps now offer augmented reality measuring features, allowing you to gauge objects virtually and compare them to known lengths.
FAQs
What is temperature in simple terms?
Temperature is a measure of how fast the tiny particles inside something are moving. Faster movement means higher temperature, slower movement means lower temperature.
How is temperature measured in science?
Science measures temperature using instruments like thermometers and sensors that respond to changes in particle motion, electrical resistance, or thermal radiation.
What is the difference between heat and temperature?
Temperature shows how hot or cold something is, while heat describes energy moving from a warmer object to a cooler one.
Why are there different temperature scales?
Different temperature scales exist because people and scientists need different reference points. Daily life favors convenience, while science requires absolute accuracy.
Why is Kelvin called an absolute temperature scale?
Kelvin starts at absolute zero, the lowest possible temperature where molecular motion is at its minimum, making it ideal for scientific calculations.
Why does Fahrenheit still exist today?
Fahrenheit remains useful because its smaller degree changes describe human comfort levels more precisely, especially for weather.
Can temperature be negative?
Yes, temperature can be negative on Celsius and Fahrenheit scales, but it still represents particle motion, not the absence of energy.
Does absolute zero mean there is no energy at all?
No, absolute zero means molecular motion is at its minimum possible level, not that all energy disappears.
Which temperature scale is most accurate?
All scales are accurate when used correctly, but Kelvin is essential for scientific equations because it measures absolute temperature.
Why do different countries use different temperature scales?
Countries use different scales due to historical choices, cultural habits, and practical needs rather than scientific limitations.
Final Thought
Temperature may seem like a simple number on a screen or a quick glance at a thermometer, but it represents something far deeper. It tells the story of molecular motion, energy balance, and how the physical world behaves at every scale. From deciding what you wear in the morning to guiding complex scientific experiments, temperature quietly influences comfort, safety, and accuracy. When you understand what temperature truly measures, why different scales exist, and how each one fits its purpose, those numbers stop feeling random. They start making sense. And once that happens, temperature becomes less of a daily annoyance and more of a powerful tool for understanding the world around you.

Jhon AJS, the author of Dimension Orbit, is an experienced blogger fascinated by the mysteries of existence. He explores every type of dimension from scientific to spiritual with clarity and creativity. Jhon’s engaging writing style invites readers to think deeper, question reality, and discover new perspectives on the universe.